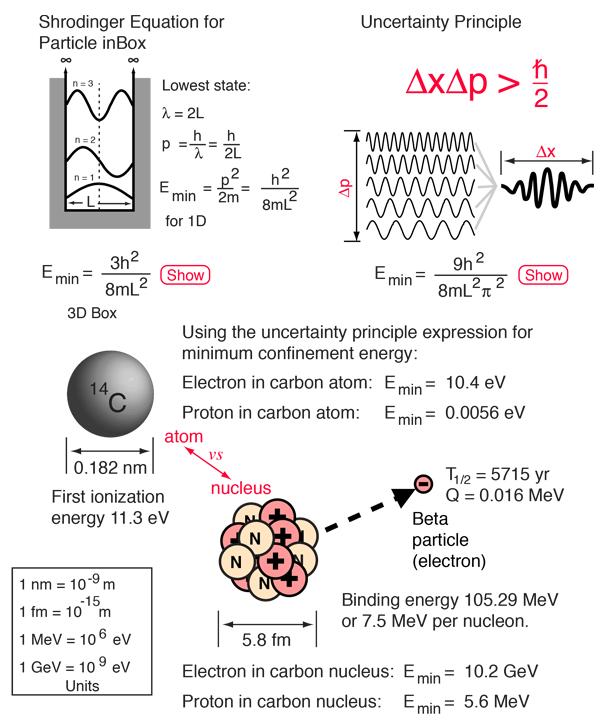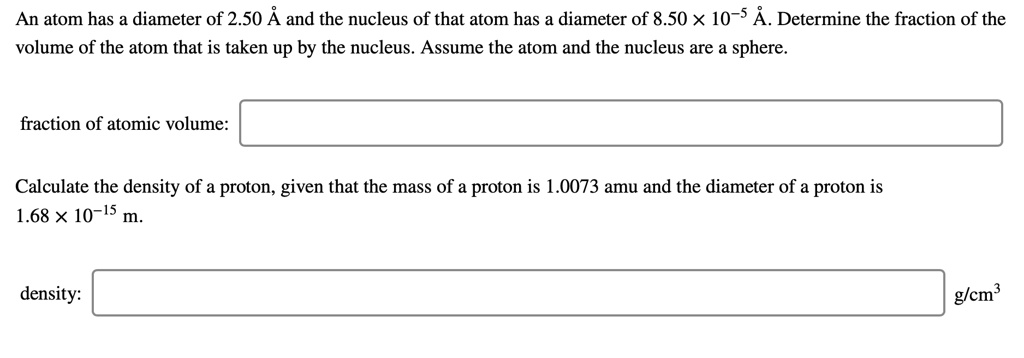
SOLVED: An atom has diameter of 2.50 A and the nucleus of that atom has diameter of 8.50 X 10-5 A. Determine the fraction of the volume of the atom that is

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises
Welcome to Chem Zipper.com......: How to calculate percentage (%) ionic character in covalent compounds?

A metal M of atomic weight 54 94 has a density of 7 42g/cc Calculate the apparent volume occupied by - Chemistry - Some Basic Concepts of Chemistry - 14546641 | Meritnation.com

A compound AB crystallises in bcc lattice with the unit cell edge length of 380 pm . Calculate1. the distance between oppositely charged Ions in the lattic.2. radius of B ^ -
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering
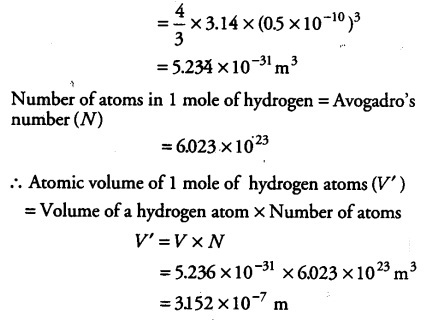





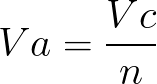



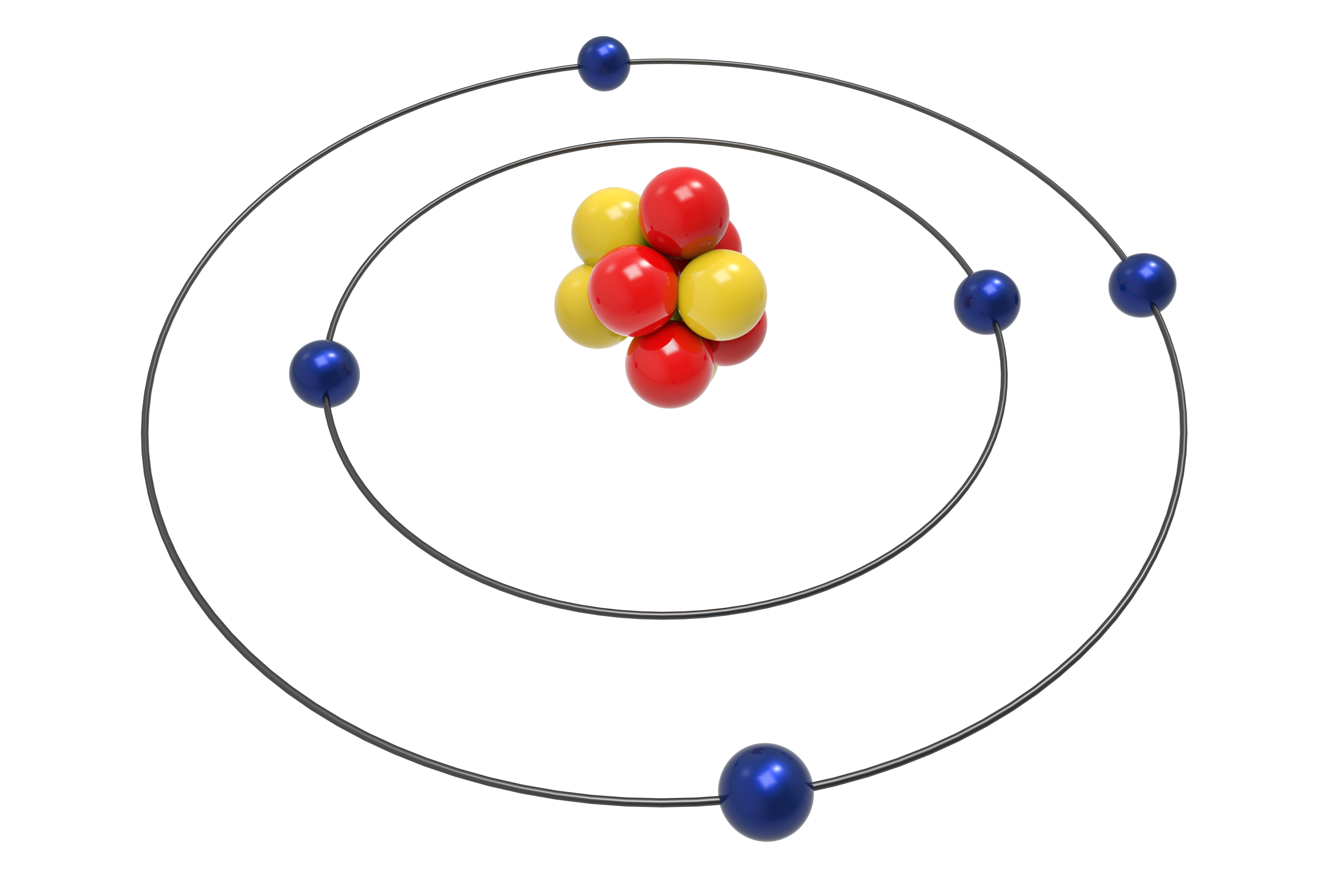

:max_bytes(150000):strip_icc()/nucleus-and-atoms-713783177-5a2bf9699e9427003730790b.jpg)

![Explained] Atomic Volume And Its Trend Explained] Atomic Volume And Its Trend](https://1.bp.blogspot.com/-En4xKfI36aI/X9nxZ-oWvDI/AAAAAAAAAgI/DpJ6--mYAzsN7Oa3cJZ0cSd-hXlT0Hh6gCLcBGAsYHQ/w1200-h630-p-k-no-nu/Atomic%2BVolume.jpg)
:max_bytes(150000):strip_icc()/surface-area-and-volume-2312247-v5-a5b14f99ba194aafa8c928231f78ee69.png)