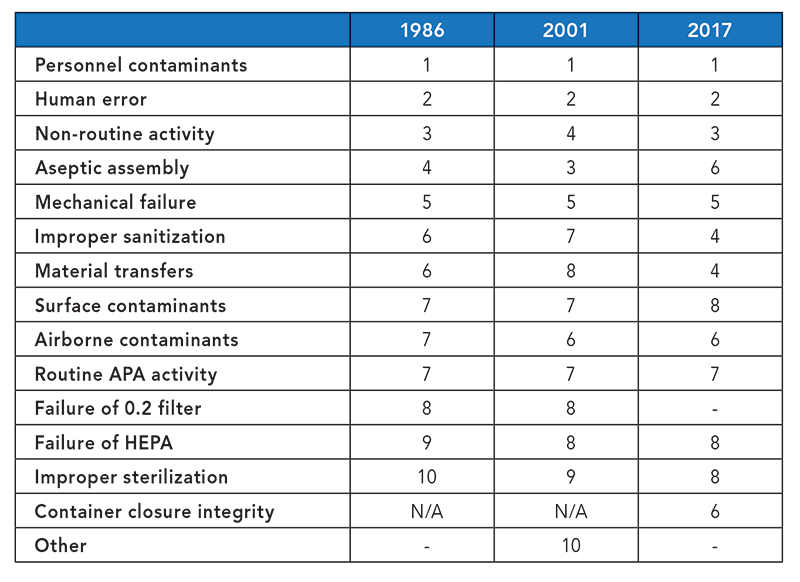
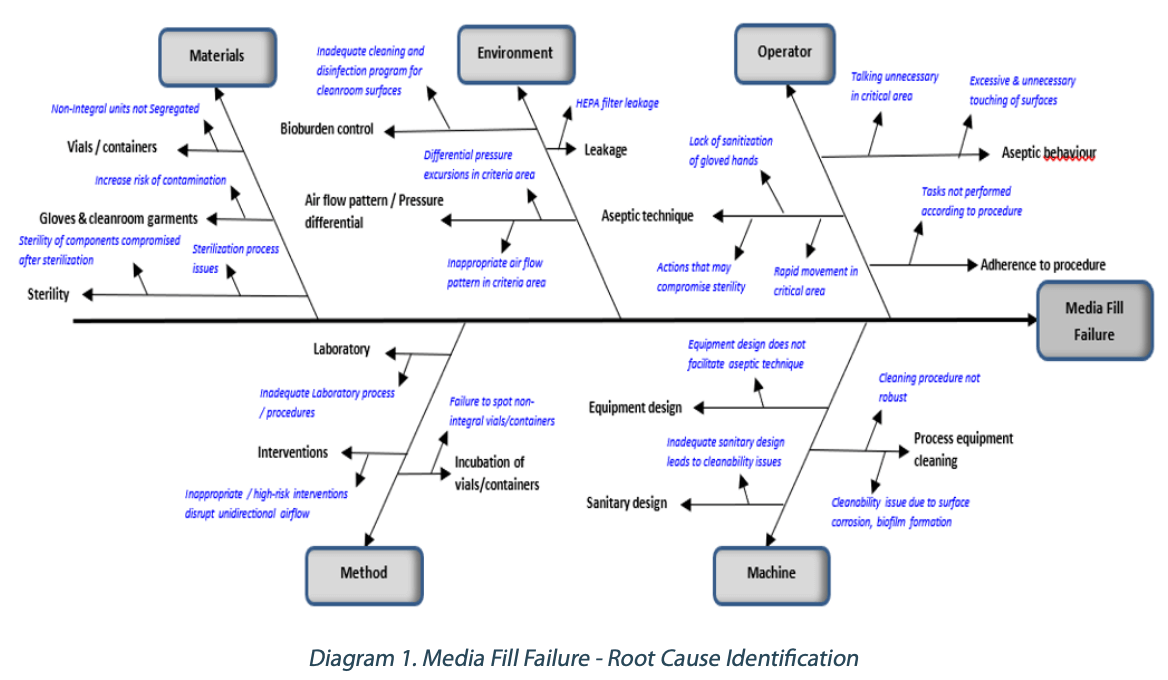
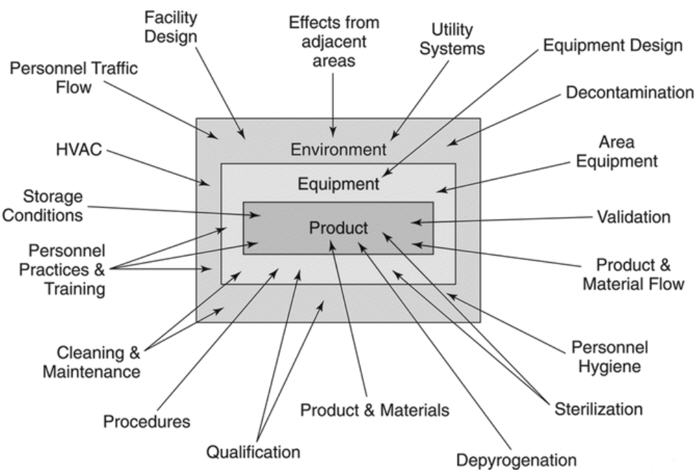
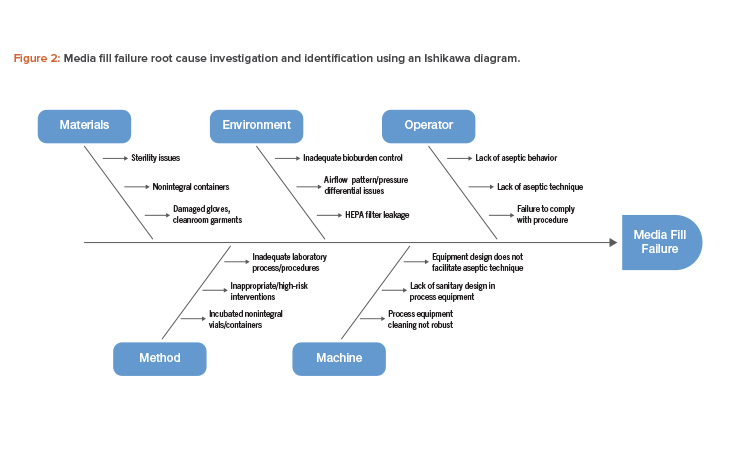
Guidance for Industry #253 - Good Manufacturing Practices for Animal Cells, Tissues, and Cell- and Tissue-Based Products

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations